Using a bubble column reactor at Karlsruher Institut für Technologie, the Liquid Metal Laboratory team is working on producing hydrogen from methane, the cheapest method, but without CO2 emissions – the problem with the cheapest method. The main product, besides hydrogen, is carbon, which, like green hydrogen, has a commercial value.
This technology would be a source of green hydrogen, using methane gas from agricultural or municipal waste and heating the pyrolysis process at temperatures as high as 1200 °C from highly concentrated sunlight, reflected and intensified in a solar field of mirrors supplying the heat from 100% solar energy. (How solar thermochemistry works)
Their papers, Natural Gas Pyrolysis in a Liquid Metal Bubble Column Reaction System—Part I_ Experimental Setup and Methods and Natural Gas Pyrolysis in a Liquid Metal Bubble Column Reaction System—Part II_ Pyrolysis Experiments and Discussion were published in the Journal Hydrogen, describing their pyrolysis process.
To make brown hydrogen from fossil sources of methane, steam methane reformers (SMRs) burn natural gas in air. The oxygen in the air combines with the carbon in methane, generating CO₂ emissions. By contrast, pyrolysis is an oxygen-free heating process, so, without oxygen, it produces no CO₂ .
“From the chemical point of view, pyrolysis is more efficient than combustion,” lead author Christoph Hofberger explained. “It’s combustion without oxygen, so you heat the methane to produce carbon and hydrogen – but no CO2 at all.”
Hofberger suggested that sunk costs are the obstacle for a hundred-year-old technology to explain why today’s brown hydrogen producers likely won’t similarly change to pyrolysis in liquid metal to at least partially reduce their CO2 emissions.
It’s one thing to solve new engineering challenges when you design a new technology from the ground up like cutting-edge solar research to solve new climate concerns, but quite another to do so as a legacy technology developed during ignorance about the climate crisis. The corrosivity of liquid metals is one such challenge.
“For pyrolysis, you need very high temperatures, for example, at least 950 degrees Celsius,” he said. “To reach a high conversion, your liquid metal temperature should be about 1200 °C. Liquid metals this hot are very corrosive, so they would have to develop and build new facilities and gain experience running them to withstand this high corrosivity. But their old technology is fully developed, so replacing everything requires high investment costs.”
For the team’s experimental work, a quartz glass-lined bubble reactor at the Karlsruhe Liquid Metals Laboratory can survive the corrosive liquid metal, liquid tin. But the glass would be too fragile at full scale to contain a large volume of extremely hot liquid metal. However, Hofberger is confident that materials scientists can solve the corrosion challenge so this new technology can scale up for commercial use.
“For example, the steel industry has really big vessels holding liquid metals,” he pointed out. “They have a coating of ceramic bricks, like, for example, silicon carbide and alumina. These are usually stable up to 1300 °C, so our temperature range wouldn’t be a big deal with something like these materials.”
Another challenge they will resolve is that the tiny holes dispersing the gases through the liquid metal are just a few millimeters in diameter, while the ceramic bricks they would be drilled through are big and thick.
The pure carbon produced can clog the walls. Previous researchers using tube reactors have seen this problem of carbon deposits. “If you use only reactor pipe for the reaction, the carbon will build up on the hot reactor wall, so after a few minutes, the reactor is completely blocked, and no gas can escape,” he said.
To solve this problem, the team used a special bubble column reactor built at their lab at the Karlsruhe Institute of Technology. This reactor system is built upon previous work and includes a quartz glass bubble column inside an electrically heated column furnace. Gas is supplied at the bottom of the reactor. The pyrolysis happens in rising gas bubbles, so there’s no direct contact with the reactor wall, so clogs can’t form.
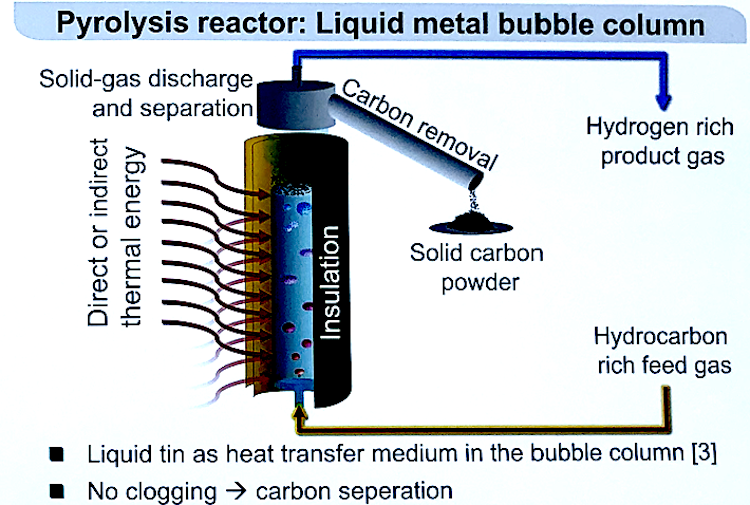
“The main benefit of using the bubble reactor is to avoid these blockages,” he said. “The carbon is formed on the surface of the liquid metal bubble, not on the hot surface area of the pipe, and each bubble rises, holding the carbon on its surface. Every time a new bubble forms, you have a new surface so no pockets can form.”
In an earlier study, the team tested a single opening for the gases to exit. This time, they have made the reactor almost four times bigger to prepare for using multiple outlets to disperse gases. The multiple openings help prevent bubbles from clumping together, which can reduce the time the gas bubbles spend in the hot liquid metal.
The gas supply system includes precise gas flow control. The gas is heated to specific temperatures during the experiments, and the flow rates and temperatures are carefully controlled.
The team also looked at more than doubling the amount of gas that goes through each opening, having found that more openings increased the production of hydrogen and carbon. After making these changes, they increased the conversion of methane into hydrogen compared to the smaller reactor used initially.
“In the last three years, there was a massive scale-up,” Hofberger said. “The single hole was state of the art for our project three years ago. The fluid dynamics of liquid metals at this temperature range is really a complex topic. There is little information and research literature on our reaction conditions about it. So we always changed only one reactor geometry at a time during the scale-up process.”
It’s been known since 2010 that methane pyrolysis needs less energy to produce hydrogen than electrolysis or combustion of methane. But back then, concentrated solar could not achieve high enough temperatures to heat the process. Now, it has become an area of concentrated solar thermochemistry research.
“Whereas the energy requirement for electrolysis is about 288 kJ per mol of H₂ , the energy requirement for methane pyrolysis is 37.4 kJ per mol of H₂ , which is not only significantly lower than for electrolysis but also lower than for steam reforming (63.3 kJ per mol of H₂ ),” states Updated hydrogen production costs and parities for conventional and renewable technologies as cited by the Karlsruhe team.
(This work did not analyze energy consumption, as this lab-scale bubbling tube reactor system is not insulated.) Hofberger said that pyrolysis in liquid metals is more energy efficient from the chemical point of view.
“If you heat the system, you need a lot of energy, but to keep the temperature constant, you only have to add the energy the reaction consumes to heat the feed gas and the heat loss to the environment,” he said. “And these can be minimized with efficient insulation and heat recovery.”
More reading:
Geißler, T.; Plevan, M.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Experimental investigation and thermo-chemical modeling of methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Int. J. Hydrogen Energy 2015, 40, 14134–14146. Available online: https://www.sciencedirect. com/science/article/pii/S0360319915022491 (accessed on 1 December 2022). [CrossRef]
Geißler, T.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Müller, G.; Rathnam, R.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Hydrogen production via methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Chem. Eng. J. 2016, 299, 192–200. Available online: https://www.sciencedirect.com/science/article/pii/S1385894716305162 (accessed on 1 December 2022). [CrossRef]
Abánades, A.; Rathnam, R.K.; Geißler, T.; Heinzel, A.; Mehravaran, K.; Müller, G.; Plevan, M.; Rubbia, C.; Salmieri, D.; Stoppel, L.; et al. Development of methane decarbonisation based on liquid metal technology for CO2-free production of hydrogen. Int. J. Hydrogen Energy 2016, 41, 8159–8167. [CrossRef]