Most industries currently burn fossil fuels for heat. But to supply heat directly from sunlight, research attention is turning to solar thermal energy storage (TES) so that heat can be delivered as consistently as combusting a fossil fuel using purely solar energy.
A solar thermal energy storage breakthrough – a novel combination of latent and sensible heat in a single tank that avoids thermocline thickening has been tested successfully at the PROMES-CNRS solar thermal research laboratory
A team at the concentrated solar thermal research laboratory at PROMES, led by Gilles Flamant, Research Director Emeritus at CNRS, has tested a new way of storing this solar heat. The results of their experiment were presented at the SolarPACES Conference in 2022: Experimental Evaluation of a Pilot-Scale Thermo- cline Thermal Energy Storage Combining Latent and Sensible Materials.
The experiment validated theoretical studies
The team combined two thermal energy storage technologies in a single tank. One is sensible heat storage that absorbs heat directly. In this case, in alumina spheres. This is paired with latent storage: a phase-changing material (PCM) contained in metal tubes. The PCM chosen in this case is a molten salt, sodium nitrate (NaNO3), which changes from liquid to solid at 306 °C. Phase-changing materials store even more heat by changing between liquid and solid at a specific temperature.
A heat transfer fluid, an oil heated by sunlight, would flow through the tank, directly heating the alumina spheres and causing the sodium nitrate in the metal tubes to change from solid to liquid during the charging, and from liquid to solid as it cools in the discharging phase.
The experiment validated three theoretical studies evaluating the combination of sensible and phase-changing heat storage. One suggested that a combined solution provides a competitive cost reduction over a PCM-only thermocline while it performs better than the sensible heat TES. Another found that 5% would be the ideal ratio of this PCM to the sensible heat storage material (slag, a waste product from steel-making). A third found a very low latent to sensible storage ratio – less than 1.5% – would be feasible.
How the researchers minimized thermocline mixing
Flamant’s team tested this dual energy storage technology in a so-called thermocline tank, where hot and cold fluids are stacked vertically in the same tank. Thermocline storage is at the cutting edge of solar thermal research because using one tank reduces the cost of thermal energy storage by about a third, as long as the thermocline region where the hot and cold meet doesn’t get too thick.
Flamant explained the physical charging and discharging process in this kind of thermal energy storage system.
“The system can be thought of as a piston, where you are pushing the hot fluid – in our case, the liquid oil – from the top to the bottom during charging and the cold fluid from the bottom to the top during discharging,” he said.
“In the discharging situation, the temperature of the fluid is decreasing when the thermocline reaches the top of the storage tank. Suppose you cannot operate the conversion cycle below a certain temperature. In that case, you can define a limit of the outlet temperature for which you have to stop the discharging because, after that threshold, you cannot convert more thermal energy to power.”
Ideally, the thermocline region where the hot and cold heat transfer fluids meet should be kept as small as possible for efficient single-tank energy storage.
So as part of the test, the PROMES team measured the thickness of the thermocline during charging and discharging. They were trying to determine the optimal temperature and velocity of oil flow to keep the thermocline from taking up too much space. So they tested charging and discharging at two different temperature ranges and three different flow rates and looked at how the thickness of the thermocline changed during the charging and discharging process.
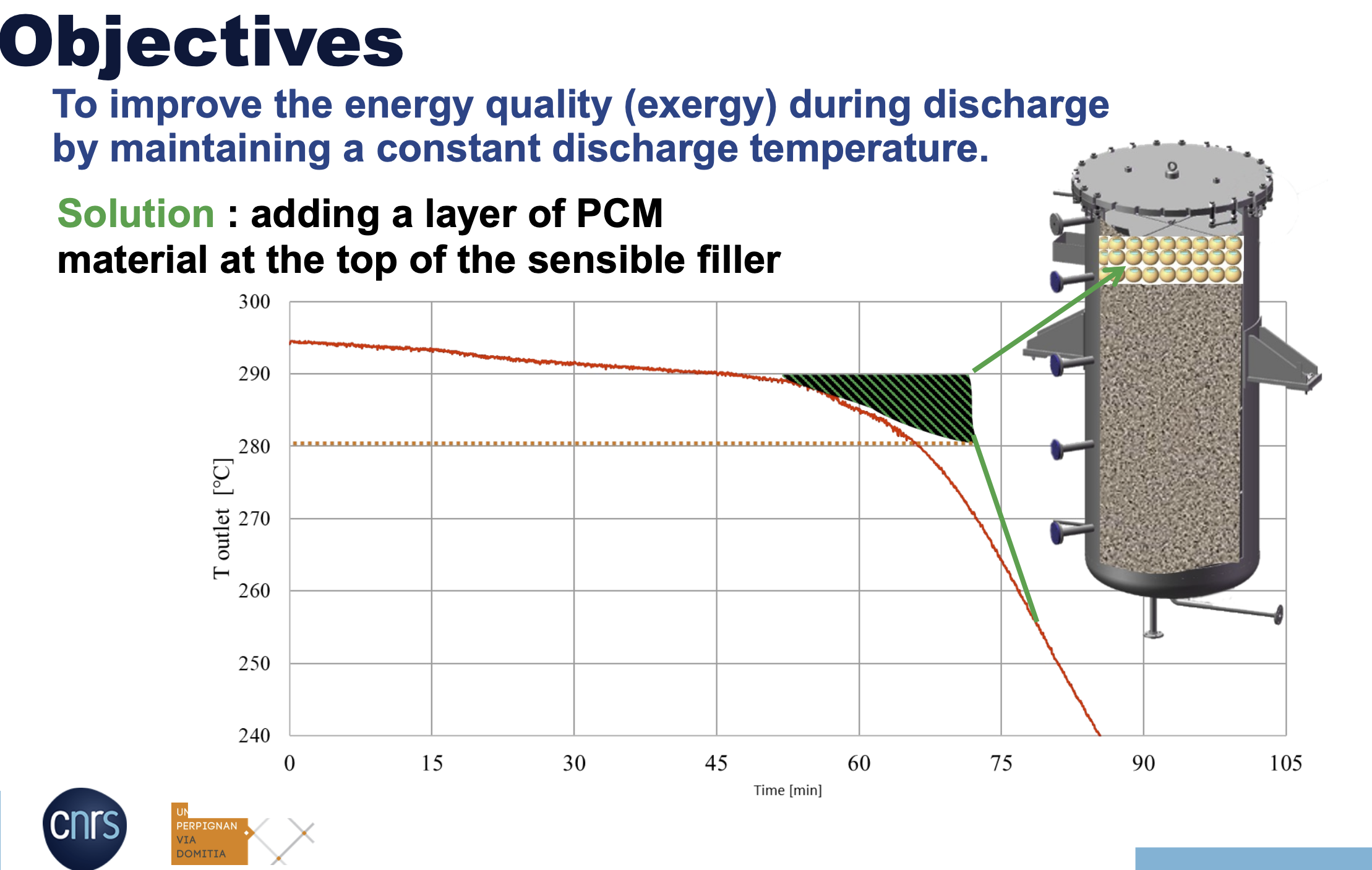
How adding the PCM layer improved the energy quality – IMAGE@PROMES-CNRS
Leveraging the “freeze” point of thesodium nitrate (NaO3)
The phase-changing point from liquid to solid for sodium nitrate is 306 °C. So they tested increasing temperatures for charging (and decreasing temperatures for discharging) that ran past that point at which sodium nitrate “freezes” or “melts.” The thermal energy storage testing rig at PROMES is the MicroSol-R parabolic trough pilot plant that is designed for this kind of temperature.
The test showed that for this type of thermal energy storage, it is best to charge and discharge slowly and at a relatively low inlet temperature.
For the charging cycle, the temperature ranges the researchers compared were 285-315 °C and 295-330 °C, while the discharging cycle was tested at temperature ranges from 315 to 220 °C and from 330 to 225 °C.
The three mass flow rates evaluated were 2600, 3000, and 3900 kg/h for charging, and for discharging, they were 1600, 2000, and 3000 kg/h. During charging, they found that the efficiency was similar at each temperature and flow rate tested.
During discharging, the maximum efficiency was at about a 2000 kg/h rate. Establishing this known optimal mass flow rate is helpful for operators of this type of thermal energy storage.
The advantage of including a phase-changing material
The 3.3 cubic meter tank contained just 337 kg of sodium nitrate as phase-changing material, but 4.66 tons of alumina spheres as sensible heat storage. So the PCM accounts for just 5% in volume of the thermal storage system.
“We need to keep the quantity of PCM to a minimum,” Flamant explained.”It is a question of cost; every time you use PCM materials, you will increase the price. On its own, the PCM is not a cost-saving solution but rather a way to optimize energy storage efficiency because of the quantity of heat you can extract from the storage at a certain temperature level. The basic question is the amount of PCM material with respect to the sensible materials you need to achieve the objective of avoiding large thermocline thickness.”
He pointed out that incorporating a PCM increases energy storage efficiency because energy can be extracted at the target temperature level during discharging. So, despite its cost, this known temperature at which a PCM “freezes” would make operation easier for plant technicians, lowering O&M costs more indirectly.
The testing rig at PROMES is set up for operation at lower temperatures. So it was suitable for testing sodium nitrate that becomes solid at 306 °C. But Flamant said choosing a different PCM with a higher temperature “switch” for its phase change would also work in a thermocline tank combining sensible and latent thermal energy storage like this test. The earlier study predicted that a PCM that changes phase at a higher temperature would have an even lower ratio to sensible storage – just 1.33%.
As for other sensible heat storage materials, Flamant suggested that some other promising materials to explore for this dual thermocline storage combining latent and sensible heat storage could be industrial wastes, steel slag – or even ceramics from asbestos.
So far, in commercial CSP plants, thermal energy storage has been in two tanks, one for the heat transfer fluid heated by the sun and the other tank (once the heat has been extracted) for the now colder fluid, which returns to the solar receiver or collector to be heated again.
This experiment did not analyze cost. But if financial analysis demonstrates the lower costs implied by this test’s efficiency increase and thermocline thickness reduction, there are significant implications for cost-effectively replacing fossil fuels with direct solar heat for industrial processes. And we would likely see CSP plants in the future use thermocline tanks that pair latent and sensible heat storage.
Susan Kraemer, solarpaces.org