Nickel ceria (Ni-CeCO₂) for solar syngas at just 700°C IMAGE@Kinetic investigation of solar chemical looping reforming of methane over Ni–CeO₂ at low temperature
Adding Nickel to Ceria can yield Solar Syngas at lower temperatures – at just 700°C
Solar thermochemistry researchers at the University of Florida and Synhelion tried a new way to generate syngas, the precursor to hydrocarbon fuels like jet fuel or diesel. Solar syngas production is an important research area for meeting climate goals. This is because producing syngas with thermochemistry heated by a solar field of heliostats can reduce carbon emissions from industry, shipping, or aviation by up to 100%, depending on the hydrogen and carbon input source.
In their paper, Kinetic investigation of solar chemical looping reforming of methane over Ni–CeO₂ at low temperature, a team led by Associate Professor Jonathan Scheffe at the Department of Mechanical and Aerospace Engineering at the University of Florida described their experiment producing syngas at a lower temperature, using a nickel catalyst deposited on ceria.
Ceria (CeO₂) is state-of-the-art for performing this type of thermochemical reaction but generally requires temperatures over 1000°C. Surface decoration of the ceria with noble metal catalysts has been shown to improve the performance compared to ceria alone. However, a more promising contender is a humble non-noble metal, nickel (Ni).
Nickel sped up the reaction – at lower temperatures.
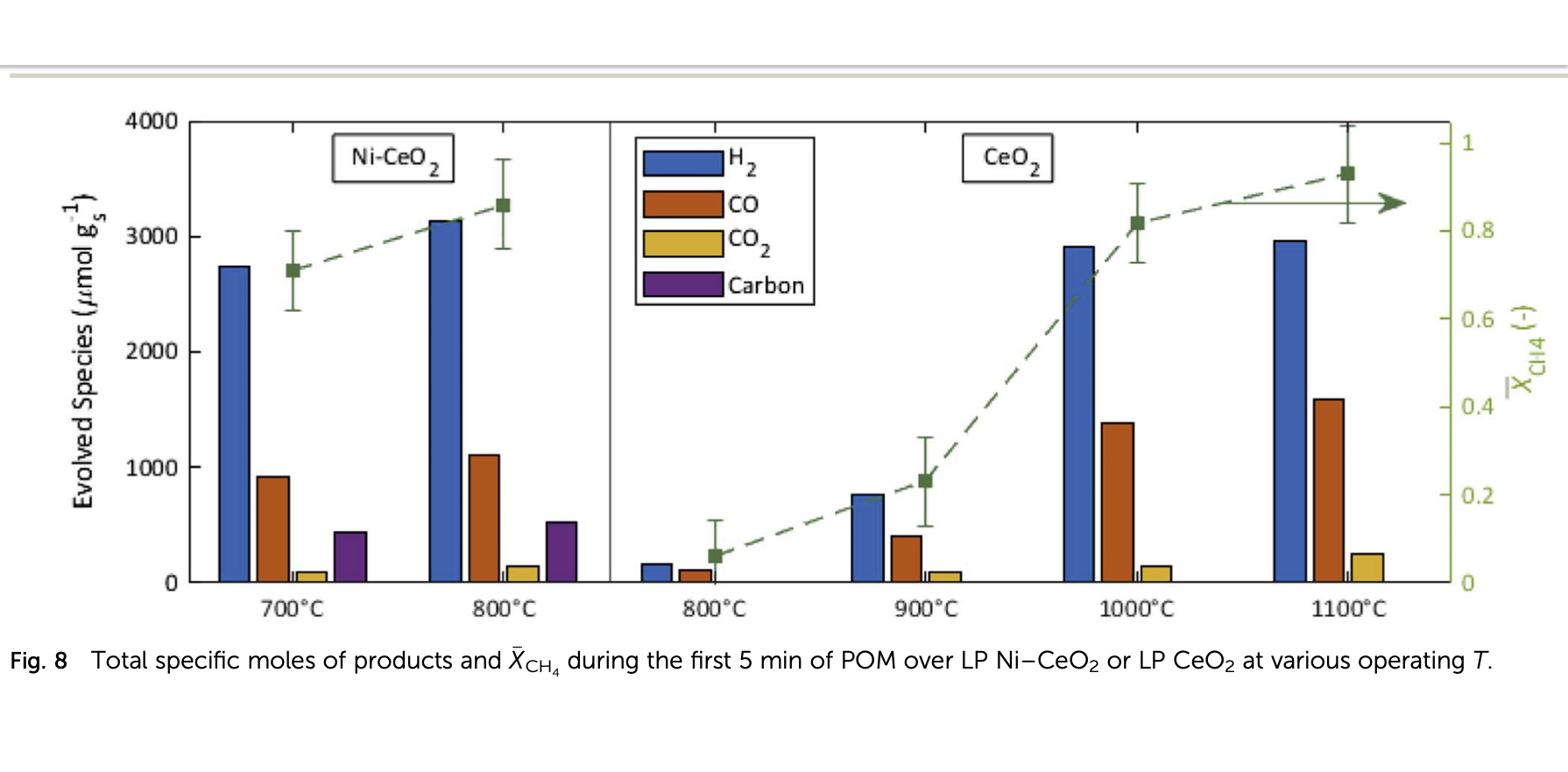
Scheffe’s team established that nickel-ceria (Ni-CeO2) can produce syngas faster than ceria alone, even at lower temperatures, with almost 100% syngas selectivity. They demonstrated that the nickel-catalyzed reaction worked at lower temperatures than ceria alone and remained stable during extended use. Ceria barely reacts at 700°C. But the Ni-CeO₂ combination they tried at 700°C performed as well as ceria by itself at 1000°C.
The paper stated: “Overall performance, including methane conversion and total syngas production, with Ni–CeO2 at low temperatures (i.e., 700 °C and 800 °C) was comparable to CeO₂ when operating at higher cycling temperatures of 1000– 1100 °C. “
This area of research has attracted other scientists
“There have been a couple of good demonstrations of chemical looping with ceria, and they showed decent efficiencies,” said Caroline Hill, the lead Ph.D. student on the experiment. “But they were at relatively higher temperatures of over 1000°C.”
The team’s result, successful syngas production at 700°C with Ni-CeO₂, has important implications for solar thermochemistry’s economics and industrial viability.
“Lower operating temperatures can provide economic benefits,” she explained. “We added nickel, a catalytic metal, to increase the kinetics and the reaction rates so that we can operate with similar conversions and selectivities but at lower temperatures. There are all kinds of economic advantages to doing so, including decreasing the capital costs, like the receiver tower and the number of heliostats required. Using lower temperatures also allows for lower materials costs for reactor components, enabling the use of common high-temperature alloys.”
Customizable CLMR can produce a range of hydrocarbon fuels
The technique they used (chemical looping methane reforming: CLMR) is a two-part process. Compared with traditional steam methane reforming (SMR), this enables precise customization in the composition of the final syngas.

“There are ideal ratios of hydrogen to carbon monoxide for different products, for diesel versus jet fuel, for example,” explained Scheffe.
“There are ideal ratios of hydrogen to carbon monoxide for different products, for diesel versus jet fuel, for example,” explained Scheffe.
“So, depending on if we want more than a two-to-one ratio of hydrogen to carbon monoxide, we add a certain amount of H₂O in the second half of the cycle, or if we want less, we can add proportionally more CO₂. So with this precise control of the final syngas composition, we can tune the ratio to whatever we want in the second half.”
The experiment with nickel-ceria (Ni-CeO₂)
Because the test was limited to just the methane reforming process, not the whole solar technology, an electric furnace simulated the heat that would be supplied from a solar field of heliostats concentrating sunlight. They heated the reactor to temperatures between 700°C and 1100°C and adjusted the flow of gases going in using special controllers. They also used a device to measure the gases coming out, including how much carbon dioxide, hydrogen, methane, and carbon monoxide were present.
They found that the syngas selectivity remained above 0.98 for the duration of the 200 cycles tested.
“You want selectivity to be as close to 100% as you can get without sacrificing other factors,” Hill said.
“So this was really good. But we also couldn’t measure selectivity with high methane conversions until we switched to a larger reactor system. This is because you have to deliver excess methane to observe the reactions in the smaller-scale experiments we did initially.”
The previous small-scale tests were run with 10 milligrams of Ni-CeO2, but Hill noted that it’s challenging to get accurate measurements in such small reactors. “But as the reactor size increases, the selectivity definition becomes more meaningful. So, in this case, using a larger packed bed reactor allowed us to obtain more accurate measurements of selectivity, which showed that the addition of nickel improved conversion without affecting the selectivity of syngas production.”
However, this test was still at a very small scale, 1-gram inside a ceramic tube. Nevertheless, such promising results suggest this should be tried on a much larger scale.
Promising results need a larger-scale experiment
“Ultimately, the results motivate more exploration at a more realistic scale,” Scheffe noted.
“Two hundred cycles was the maximum amount that we’ve ever done. But in a real process, these materials will have to be cycled daily for up to a few decades, right? Probably 100 times a day or so. So several orders of magnitude more cycles need to be investigated. But based on the stability at 200 cycles, the high conversions, the high selectivities, all suggest that this should result in high efficiencies when integrated into a real solar reactor.”
The University of Florida does have access to a high-flux solar simulator that can test reactors up to 10 kW thermal input. However, more funding is needed to do experiments on a larger scale.
Scheffe approached the ETH spin-off, Synhelion, who funded the current experimentation with Ni-CeO₂ and chemical looping methane reforming because they are already working towards commercializing the solar production of aviation fuel with ceria. To scale up the current experimental work, Scheffe is trying to source funding from various U.S. federal agencies and potentially other well-established industries that need decarbonizing.
“Two more potential industries I could imagine would be the fertilizer industry,” he said. “Hydrogen is the biggest and most important feedstock for making ammonia which is important for the fertilizer industry. And the cement industry, where they’re generating a lot of CO₂. They are responsible for almost 8% of the global CO₂ emissions.”