In this solar technology, highly concentrated solar energy directly heats a solar reactor and drives a thermochemical process, using carbon dioxide and water as the raw materials to produce syngas, a mixture of hydrogen and carbon monoxide. The syngas can be converted to liquid transportation fuels using established technologies such as Fischer-Tropsch.
Solar reactor used for the heat recovery test at ETH Zürich. IMAGE@A.Lidor
The largest energy loss in the process is the sensible heat required to heat up the reactive materials to temperatures over 1500 °C at each cycle. But so far, no research has successfully incorporated heat recovery to recoup the heat lost during solar thermochemistry.
“So this remained one of the open questions,” said Dr. Alon Lidor, a Senior Research Associate at ETH Zürich, working under Prof. Aldo Steinfeld.
“And there have been a lot of theoretical ideas over the years, all kinds of heat recovery methods – but not to actually go to the lab, build a device, and try to make it work.”
Now Lidor’s ETH team has engineered a way to recover this lost heat. The team demonstrated heat recovery at the 4 kW lab scale under concentrated thermal radiation conditions. Their results were published at Applied Energy in High-temperature heat recovery from a solar reactor for the thermochemical redox splitting of H2O and CO2.
Solar fuels made this way are a clean alternative to fossil fuels
Solar thermochemistry-driven processes to make syngas for drop-in solar aviation fuel have taken off in the last five years, with successful demonstrations and lab research spin-offs like Synhelion starting to go commercial.
Solar thermochemical research is crucial for climate change mitigation goals because long-haul aviation and shipping require liquid fuels with high specific energy. As such, these sectors are difficult to decarbonize. Today, these sectors use fossil-based fuels such as jet fuel and diesel.
The solar reactor lab set-up
The ETH team build on a successful thermochemical process using ceria in a solar reactor’s two-step reduction-oxidation (redox) cycle. In the first step, a thermal reduction occurs using the energy from the highly concentrated sunlight, heating the reactor to temperatures of 1500°C and above.
At the same time, a vacuum pump reduces the pressure in the reactors to about ten millibars. The reactive material (or redox material) used is ceria, an abundant ceramic with proven stability. It is installed inside the reactor as porous foam-like bricks to allow the solar radiation to penetrate while increasing the surface area for the chemical reaction.
Under thermal reduction, oxygen is released from the ceria. Once reduction is finished, the solar radiation is stopped, and the reactor passively cools down to below 1000 °C, ejecting the heat. Water and carbon dioxide are introduced. They react with the ceria, re-oxidizing it to produce hydrogen and carbon monoxide before the next cycle begins. ETH has previously demonstrated the entire production chain to generate aviation fuel (kerosene).
The lab scale 4 kW solar reactor they used is an octagonal insulated cavity in a stainless steel cylindrical outer shell. The 40 mm aperture at the front receiving heat as solar flux is sealed by a four mm-thick quartz window. The inner cavity is roughly 100 mm in diameter and 75 mm deep. The ceria bricks are 35 mm thick and have a total mass of 2788 g, creating the cavity inside.
“We said okay, let’s take a successful working reactor and try to tailor thermal energy storage and heat recovery to it,” Lidor explained. “You can think, of course, how to improve and modify the reactor itself. But doing this allowed us to go directly to the lab and not be limited to a theoretical study.“
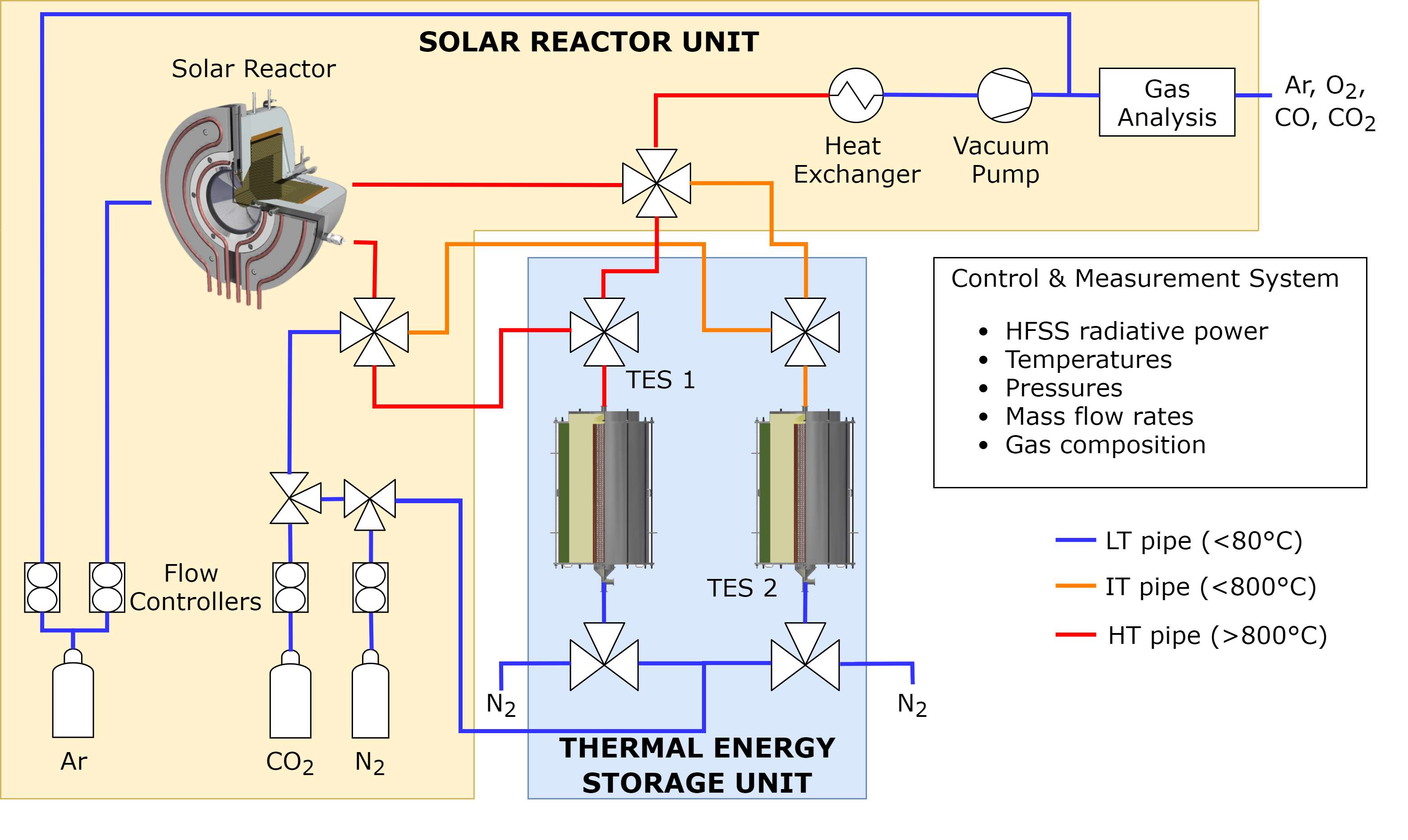
Schematic for the ETH Zurich solar reactor with heat recovery IMAGE from the paper High-temperature heat recovery from a solar reactor for the thermochemical redox splitting of H₂ O and CO₂ .
How the solar reactor heat-recovery takes place
Next to the solar reactor where the redox process takes place, two thermal energy storage units are placed. The heat recovered from the reactor is stored in them and used in the reactor upon demand.
The heat recovery works by adding two extra steps to the cycle. At the end of the reduction step, the reactor is at its highest temperature, 1500 °C. A heat extraction step is added following the reduction, in which a heat transfer fluid extracts the high-temperature heat from the reactor. This heat is stored in the large thermal energy storage unit. Following the extraction, the oxidation step follows as usual, identical to the case without heat recovery.
Once oxidation is finished, the heat stored in the large thermal energy storage unit is recovered into the reactor by flowing cold air through it. Since the air leaves the solar reactor with a few hundred degrees of residual heat, this is recovered in the small thermal energy storage unit. The heat recovery partially heats the solar reactor, so the rest of the reduction step and heating to 1500 °C is performed using concentrated sunlight, thus starting the next cycle.
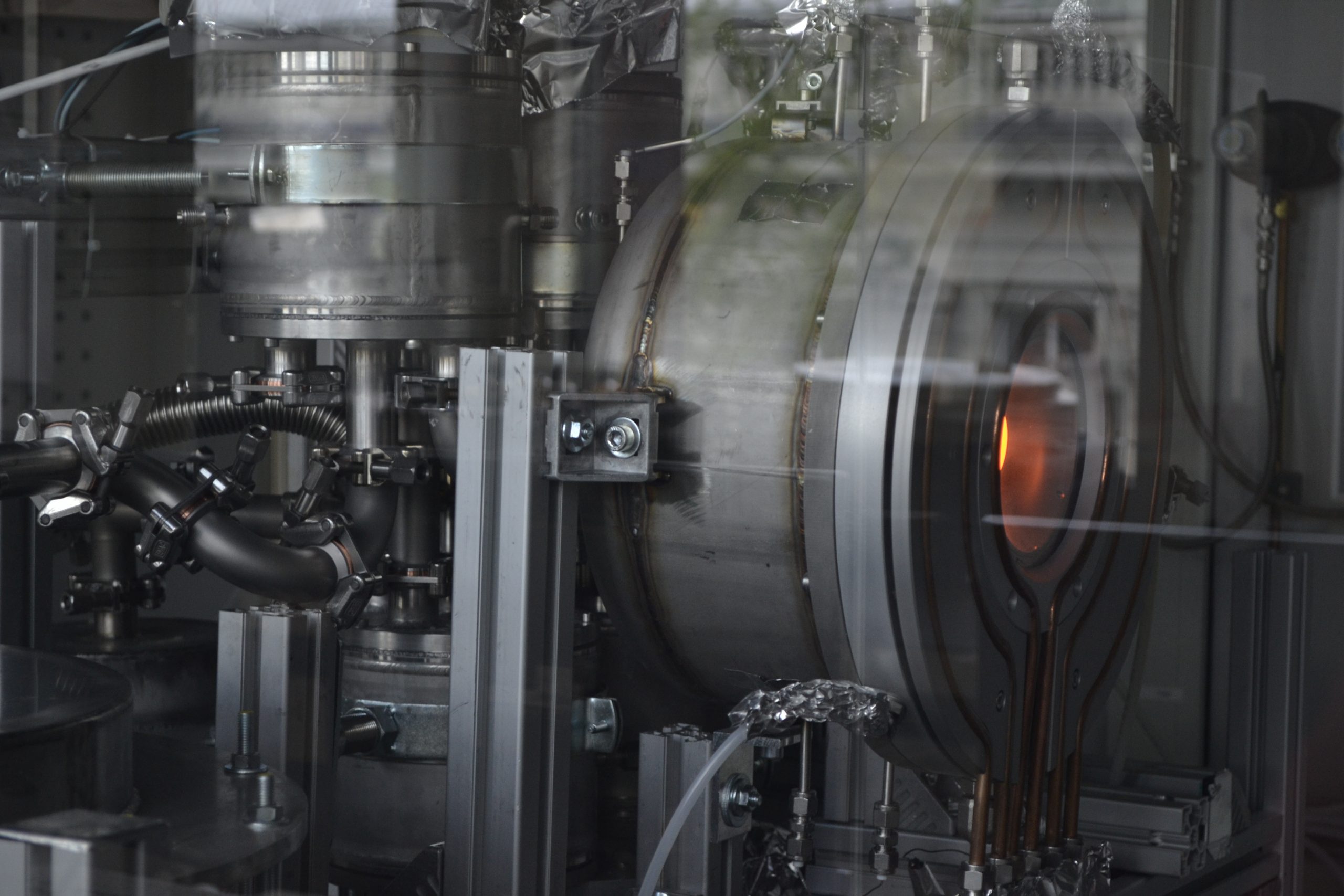
The thermal storage tank and the heat-recovery unit beneath it on the left adjacent to the solar reactor which receives solar heat to perform the thermochemistry on the right IMAGE@A. Lidor
What the thermal storage and the heat-recovery look like
Both thermal energy storage units are insulated cylindrical tanks holding vertical tubes with a packed bed of small porous alumina spheres (up to 5 mm) to store the heat with the heat transfer fluid flowing down through the tubes. They are closed by flanges on the top and bottom, with thermocouples inserted at various locations to measure the temperature. The hot fluid enters from the top when the storage tank is charged, leaving cold at the bottom. During discharge, the direction is reversed, with cold fluid coming from the bottom and leaving the storage tank from the top at a high temperature.
“The vertical design has some benefits; when the high-temperature side is on the top, the buoyancy effects are in our favor,” he commented.
“The vertical design is also good for mechanical stability. Some experiments in the lab before my time used horizontal tubes in the tank, but they cracked. There are a lot of stresses, and in general ceramic materials are very brittle. There are thermal and mechanical stresses when you have a high temperature at one end and a lower temperature at the other, which is the case in our setup.”
For the heat transfer fluid, to carry the concentrated solar heat through the system, the team chose nitrogen partly for its ease of use but also because nitrogen does not corrode metal or react with the ceria.
“We recover the heat from the hot redox material directly, so it comes in direct contact with the ceria,” Lidor explained. “We want to extract the heat, but we don’t want to inadvertently re-oxidize it.”
What the experiment achieved
The team demonstrated the operation of the complete setup and studied its behavior, and demonstrated extracting up to 70% of the sensible heat at 1267°C from the solar reactor. Unfortunately, heat losses between the reactor and thermal energy storage unit prevented charging it at temperatures high enough to demonstrate heat recuperation back into the reactor. However, based on experimentally measured values for each step, they were able to calculate the performance of the setup, as well as study its scale-up potential.
“This showed that if we can use heat recovery and minimize those sensible heat losses, which are large in a small lab-scale setup but reduces with size, this can potentially push the efficiency,” he said.
“And based on our experimental measurements, not on a purely theoretical model, we showed that at a larger scale, we can go to double-digit solar fuels efficiency, which would be a record that no one has achieved yet.”



